Abstract
Background: Unusual cases of thrombocytopenia and thrombosis were reported shortly after the introduction of the COVID-19 vaccine. In most cases, symptoms started between 5 and 30-day after vaccination. Most of these patients have positive anti-platelet factor 4 (PF4) antibodies and platelet activation assay, which is believed to be responsible for thrombosis and thrombocytopenia. Unlike heparin-induced thrombocytopenia (HIT), these patients have no recent heparin exposure, and anti-PF4 antibodies are believed to be induced by vaccination against COVID-19.
The incidence of VITT varies among studies ranging from 25,000-250,000 but is associated with high mortality. Treatment of VITT is extrapolated from HIT due to similarities between the two disorders. Anticoagulation with heparin is discouraged, but observational data suggest that anticoagulation with heparin is safe in this patient. High-dose intravenous immunoglobulin (IVIG) is also used in cases of severe VITT. The use of IVIG is associated with rapid recovery of platelet count and inhibition of platelet activation assays. In this meta-analysis, we intend to investigate the impact of anticoagulation and IVIG on thromboembolism and mortality among patients with vaccine-induced thrombotic thrombocytopenia.
Methods: We searched OVID and PubMed from the inception of the database to July 15, 2022, for observational studies reporting the incidence of thrombosis and mortality among patients with VITT. Case reports and review articles were excluded. Statistical analysis was performed with MedCalc. Both fixed and random effect model was used for pooled analysis.
Results
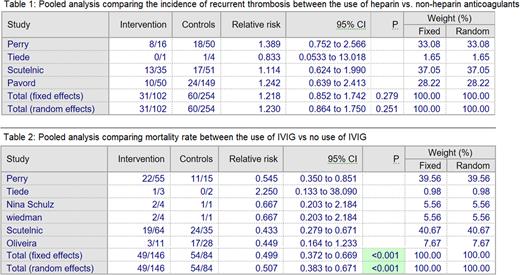
Eight observational studies, including 495 patients, were included in the meta-analysis. The mean age of the patient ranged from 35 to 61 years; the majority were females. Most of the patients received anticoagulation (419/495). Pooled analysis shows no difference in the incidence of thrombosis among patients who receive anticoagulation with heparin compared to the non-heparin anticoagulants with a relative risk of 1.2 (95% confidence interval of 0.85 to 1.74). 304 patients (61%) received IVIG out of 495 patients. Mortality data were available for 230 patients. Pooled analysis shows that IVIG was associated with a lower mortality risk with a relative risk of 0.49 (95% confidence interval 0.37 to 0.67).
Conclusion Our analysis shows that IVIG reduces mortality among patients with VITT. There was no significant difference in the incidence of thrombosis between heparin and non-heparin anticoagulants.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.